- Hypothalamus and Pituitary Gland
- Metabolic Impacts of Discontinuation and Resumption of Recombinant Human Growth Hormone Treatment during the Transition Period in Patients with Childhood-Onset Growth Hormone Deficiency
-
Yun Jeong Lee, Yunha Choi, Han-Wook Yoo, Young Ah Lee, Choong Ho Shin, Han Saem Choi, Ho-Seong Kim, Jae Hyun Kim, Jung Eun Moon, Cheol Woo Ko, Moon Bae Ahn, Byung-Kyu Suh, Jin-Ho Choi
-
Endocrinol Metab. 2022;37(2):359-368. Published online April 25, 2022
-
DOI: https://doi.org/10.3803/EnM.2021.1384
-
-
4,430
View
-
184
Download
-
3
Web of Science
-
3
Crossref
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material PubReader PubReader  ePub ePub
- Background
Discontinuing growth hormone (GH) treatment during the transition to adulthood has been associated with adverse health outcomes in patients with childhood-onset growth hormone deficiency (CO-GHD). This study investigated the metabolic changes associated with interrupting GH treatment in adolescents with CO-GHD during the transition period.
Methods
This study included 187 patients with CO-GHD who were confirmed to have adult GHD and were treated at six academic centers in Korea. Data on clinical parameters, including anthropometric measurements, metabolic profiles, and bone mineral density (BMD) at the end of childhood GH treatment, were collected at the time of re-evaluation for GHD and 1 year after treatment resumption.
Results
Most patients (n=182, 97.3%) had organic GHD. The median age at treatment discontinuation and re-evaluation was 15.6 and 18.7 years, respectively. The median duration of treatment interruption was 2.8 years. During treatment discontinuation, body mass index Z-scores and total cholesterol, low-density lipoprotein, and non-high-density lipoprotein (HDL) cholesterol levels increased, whereas fasting glucose levels decreased. One year after GH treatment resumption, fasting glucose levels, HDL cholesterol levels, and femoral neck BMD increased significantly. Longer GH interruption (>2 years, 60.4%) resulted in worse lipid profiles at re-evaluation. The duration of interruption was positively correlated with fasting glucose and non-HDL cholesterol levels after adjusting for covariates.
Conclusion
GH treatment interruption during the transition period resulted in worse metabolic parameters, and a longer interruption period was correlated with poorer outcomes. GH treatment should be resumed early in patients with CO-GHD during the transition period.
-
Citations
Citations to this article as recorded by  - Ghrelin regulating liver activity and its potential effects on liver fibrosis and Echinococcosis
Jiang Zhu, Tanfang Zhou, Meng Menggen, Kalibixiati Aimulajiang, Hao Wen
Frontiers in Cellular and Infection Microbiology.2024;[Epub] CrossRef - Relationship between the Stimulated Peak Growth Hormone Level and Metabolic Parameters in Children with Growth Hormone Deficiency
Seong Yong Lee
The Ewha Medical Journal.2023;[Epub] CrossRef - Dyslipidaemia and growth hormone deficiency – A comprehensive review
Matthias Hepprich, Fahim Ebrahimi, Emanuel Christ
Best Practice & Research Clinical Endocrinology & Metabolism.2023; 37(6): 101821. CrossRef
- Adrenal Gland
Big Data Articles (National Health Insurance Service Database)
- Epidemiology and Long-Term Adverse Outcomes in Korean Patients with Congenital Adrenal Hyperplasia: A Nationwide Study
-
Jung Hee Kim, Sunkyu Choi, Young Ah Lee, Juneyoung Lee, Sin Gon Kim
-
Endocrinol Metab. 2022;37(1):138-147. Published online February 28, 2022
-
DOI: https://doi.org/10.3803/EnM.2021.1328
-
-
3,356
View
-
149
Download
-
8
Web of Science
-
10
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Previous studies on the epidemiology and complications of congenital adrenal hyperplasia (CAH) were conducted in Western countries and in children/adolescents. We aimed to explore the epidemiology of CAH, as well as the risk of comorbidities and mortality, in a Korean nationwide case-control study.
Methods
CAH patients (n=2,840) were included between 2002 and 2017 from the National Health Insurance Service database and the Rare Intractable Disease program. CAH patients were compared, at a 1:10 ratio, with age-, sex-, and index year-matched controls (n=28,400).
Results
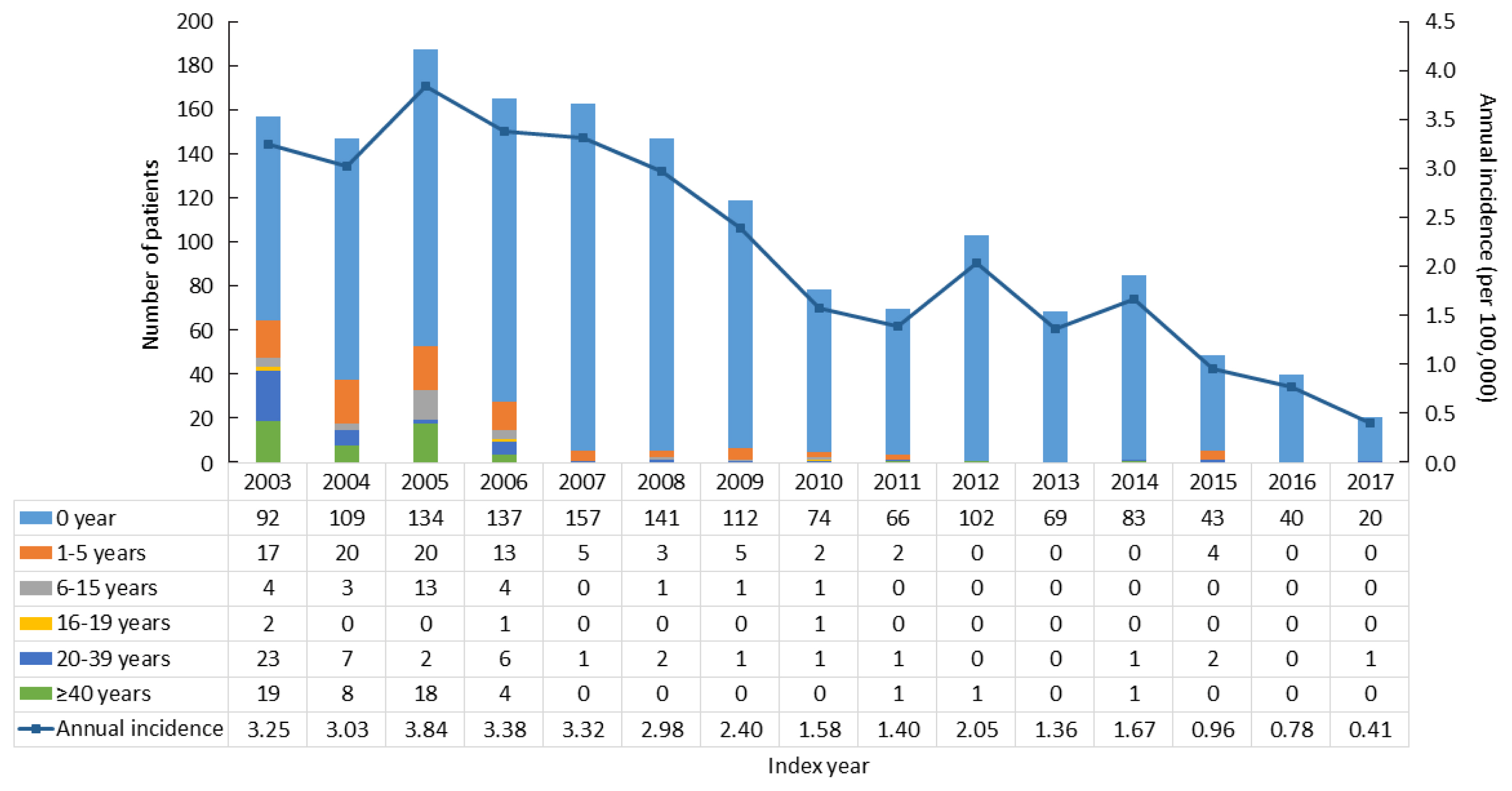
The point prevalence of CAH patients in Korea was 1 in 18,745 persons in 2017. The annual incidence rate declined between 2003 and 2017 from 3.25 to 0.41 per 100,000 persons. CAH patients were at elevated risk for cardiovascular disease (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.4 to 1.9), stroke (OR, 1.7; 95% CI, 1.3 to 2.0), diabetes mellitus (OR, 2.8; 95% CI, 2.6 to 3.1), dyslipidemia (OR, 2.4; 95% CI, 2.2 to 2.6), and psychiatric disorders (OR, 1.5; 95% CI, 1.3 to 1.6). Fracture risk increased in CAH patients aged over 40 years (OR, 1.4; 95% CI, 1.1 to 1.7). CAH patients were at higher risk of mortality than controls (hazard ratio, 1.6; 95% CI, 1.3 to 2.0).
Conclusion
Our nationwide study showed a recent decline in the incidence of CAH and an elevated risk for cardiovascular, metabolic, skeletal, and psychiatric disorders in CAH patients. Lifelong management for comorbidity risk is a crucial component of treating CAH patients.
-
Citations
Citations to this article as recorded by  - Hyperandrogenism and Cardiometabolic Risk in Pre- and Postmenopausal Women—What Is the Evidence?
Angelica Lindén Hirschberg
The Journal of Clinical Endocrinology & Metabolism.2024; 109(5): 1202. CrossRef - Predictors of Cardiovascular Morbidities in Adults With 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia
Suranut Charoensri, Richard J Auchus
The Journal of Clinical Endocrinology & Metabolism.2024; 109(3): e1133. CrossRef - Case report: Development of central precocious puberty in a girl with late-diagnosed simple virilizing congenital adrenal hyperplasia complicated with Williams syndrome
Eun Young Joo, Myung Ji Yoo, Su Jin Kim, Woori Jang, Ji-Eun Lee
Frontiers in Endocrinology.2024;[Epub] CrossRef - Анализ распространенности и заболеваемости надпочечниковой недостаточностью в мире
М. Ю. Юкина, Н. Ф. Нуралиева, Е. А. Трошина
Ateroscleroz.2023; 18(4): 426. CrossRef - Big Data Research in the Field of Endocrine Diseases Using the Korean National Health Information Database
Sun Wook Cho, Jung Hee Kim, Han Seok Choi, Hwa Young Ahn, Mee Kyoung Kim, Eun Jung Rhee
Endocrinology and Metabolism.2023; 38(1): 10. CrossRef - Long-term cardiometabolic morbidity in young adults with classic 21-hydroxylase deficiency congenital adrenal hyperplasia
Beatrice Righi, Salma R. Ali, Jillian Bryce, Jeremy W. Tomlinson, Walter Bonfig, Federico Baronio, Eduardo C. Costa, Guilherme Guaragna-Filho, Guy T’Sjoen, Martine Cools, Renata Markosyan, Tania A. S. S. Bachega, Mirela C. Miranda, Violeta Iotova, Henrik
Endocrine.2023; 80(3): 630. CrossRef - Serum steroid profile captures metabolic phenotypes in adults with classic congenital adrenal hyperplasia
Chang Ho Ahn, Jaeyoon Shim, Han Na Jang, Young Ah Lee, Sang-Won Lee, Man Ho Choi, Jung Hee Kim
The Journal of Steroid Biochemistry and Molecular Biology.2023; 234: 106374. CrossRef - Long‐term health consequences of congenital adrenal hyperplasia
Riccardo Pofi, Xiaochen Ji, Nils P. Krone, Jeremy W. Tomlinson
Clinical Endocrinology.2023;[Epub] CrossRef - Multiplexed Serum Steroid Profiling Reveals Metabolic Signatures of Subtypes in Congenital Adrenal Hyperplasia
Jaeyoon Shim, Chang Ho Ahn, Seung Shin Park, Jongsung Noh, Chaelin Lee, Sang Won Lee, Jung Hee Kim, Man Ho Choi
Journal of the Endocrine Society.2023;[Epub] CrossRef - Long-Term Outcomes of Congenital Adrenal Hyperplasia
Anna Nordenström, Svetlana Lajic, Henrik Falhammar
Endocrinology and Metabolism.2022; 37(4): 587. CrossRef
- Clinical Study
- Effects of Maternal Iodine Status during Pregnancy and Lactation on Maternal Thyroid Function and Offspring Growth and Development: A Prospective Study Protocol for the Ideal Breast Milk Cohort
-
Young Ah Lee, Sun Wook Cho, Ho Kyung Sung, Kyungsik Kim, Young Shin Song, Sin Je Moon, Jung Won Oh, Dal Lae Ju, Sooyeon Choi, Sang Hoon Song, Gi Jeong Cheon, Young Joo Park, Choong Ho Shin, Sue K. Park, Jong Kwan Jun, June-Key Chung
-
Endocrinol Metab. 2018;33(3):395-402. Published online September 18, 2018
-
DOI: https://doi.org/10.3803/EnM.2018.33.3.395
-
-
4,928
View
-
84
Download
-
1
Web of Science
-
2
Crossref
-
 Abstract Abstract
 PDF PDF PubReader PubReader  ePub ePub
- Background
Iodine is an intrinsic element of thyroid hormone, which is essential for childhood growth and development. The Ideal Breast Milk (IBM) cohort study aims to evaluate the effects of maternal iodine status during pregnancy and lactation on maternal thyroid function, offspring growth and development, and offspring thyroid function. MethodsThe IBM cohort study recruited pregnant women from Seoul National University Hospital between June 2016 and August 2017, followed by enrollment of their offspring after delivery. For the maternal participants, iodine status is evaluated by urinary iodine concentration (UIC) and dietary records in the third trimester and at 3 to 4 weeks and 12 to 15 months postpartum. For the child participants, cord blood sampling and UIC measurements are performed at birth. At 3 to 4 weeks of age, UIC and breastmilk iodine concentrations are measured. At 12 to 15 months of age, growth and development are assessed and measurements of UIC, a thyroid function test, and ultrasonography are performed. ResultsA total of 198 pregnant women in their third trimester were recruited. Their mean age was 35.1±3.5 years, and 78 (39.4%) of them were pregnant with twins. Thirty-three (16.7%) of them had a previous history of thyroid disease. ConclusionKorea is an iodine-replete area. In particular, lactating women in Korea are commonly exposed to excess iodine due to the traditional practice of consuming brown seaweed soup postpartum. The study of the IBM cohort is expected to contribute to developing guidelines for optimal iodine nutrition in pregnant or lactating women.
-
Citations
Citations to this article as recorded by  - High intakes of iodine among women during pregnancy and the postpartum period has no adverse effect on thyroid function
Dal Lae Ju, Sun Wook Cho, Chae Won Chung, Young Ah Lee, Gi Jeong Cheon, Young Joo Park, Choong Ho Shin, Jong Kwan Jun, June-Key Chung, Sue K. Park, YoonJu Song
European Journal of Nutrition.2023; 62(1): 239. CrossRef - Associations between maternal thyroid function in pregnancy and child neurodevelopmental outcomes at 20 months in the Seychelles Child Development Study, Nutrition Cohort 2 (SCDS NC2)
Anna M. Monaghan, Maria S. Mulhern, Emeir M. Mc Sorley, J.J. Strain, Theresa Winter, Edwin van Wijngaarden, Gary J. Myers, Philip W. Davidson, Conrad Shamlaye, Jude Gedeon, Alison J. Yeates
Journal of Nutritional Science.2021;[Epub] CrossRef
|